Answer:
m = 671 grams
Step-by-step explanation:
Given that,
No of moles, n = 4.9
Molar mass of Barium, M = 137 g
Mass divided by molar mass is equal to no of moles. It can be given by the formula as follows :
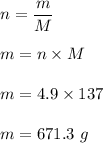
or
m = 671 grams
So, the total mass of the sample of Barium is 671 grams.