Answer:
A) oxygen moles/sec = 48 * 10 ^-14 mol/sec
B) mass of oxygen = 1536*10^-14 grams
Step-by-step explanation:
cross sectional area of cylinder = 2cm^2 = 2 * 10^-4 m^2
length of cylinder = 5 cm = 0.05 m
concentration at one end = 0.2 mol m^-3
concentration falls linearly at other end = 0.05 mol m^-3
Diffusion constant of oxygen = 8 * 10^-10 m^2 s^-1
A ) Number of moles of oxygen passing every second
N = A * D *
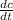 ------- ( 1 )
------- ( 1 )
A = area , D = diffusion constant,
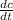 = rate of change of diffusion
= rate of change of diffusion
dc /dt = ( 0.2 - 0.05 ) / 0.05 = 3 mol m^-4
back to the equation
N = ( 2 * 10^-4 ) * ( 8 * 10^-10 ) * ( 3 ) = 48 * 10 ^-14 mol/sec
B) Mass of oxygen passing down the cylinder each second
This can be obtained by converting : 48 * 10^-14 moles to grams
1 mole of oxygen = 32 grams of oxygen
therefore : 48 * 10^-14 moles of oxygen = ( 32 * (48*10^-14) = 1536*10^-14 grams