Answer:
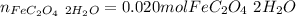
Step-by-step explanation:
Hello.
In this case, since the chemical reaction for the first step is:
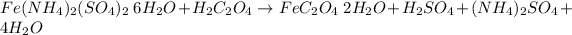
Whereas we can see a 1:1 molar ratio between FeC2O4·2H2O(s) and H2C2O4, thus, we compute the moles of yielded FeC2O4·2H2O(s) in the first step as shown below:
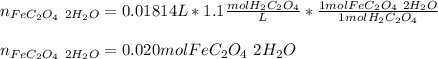
Best regards.