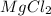Answer:
The the empirical formula for magnesium chloride based on this experiment will be
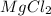
Step-by-step explanation:
Given that,
Mass of Mg = 0.50 g
Mass of magnesium chloride found = 1.99 g
Let the formula of magnesium chloride be
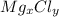
We know that,
Molar mass of Mg= 24 g/mol
Molar mass of magnesium chloride = (24x+35.5y) g/mol
We need to calculate the moles of Mg
Using formula of moles
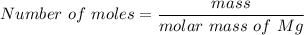
Put the value into the formula
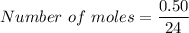
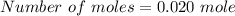
We need to calculate the mole of magnesium chloride
Using formula of moles
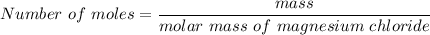
Put the value into the formula
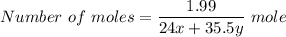
The reaction will be,
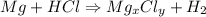
We need to calculate the value of x and y
Using number of moles of Mg in reactant and product
Moles of Mg atom in reactant=Moles of Mg atom in product
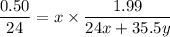
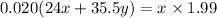
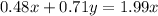
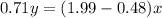
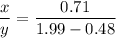
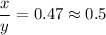
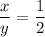
Hence, The the empirical formula for magnesium chloride based on this experiment will be