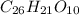Answer:
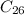
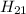
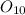
Step-by-step explanation:
We are given the following percentages:
Carbon ------ 62.4%
Hydrogen ------------ 4.19%
Oxygen ---------------- 33.2%
Now, let's take a 100g sample of this compound
since percentages are calculated out of 100, you will notice that the mass of the elements in the sample is the same as their percent composition
So we have:
Carbon -------- 62.4 grams
Hydrogen ----------- 4.19 grams
Oxygen ------------ 33.2 grams
With this information, we can calculate the number of moles of the respective elements in the given compound
Moles of Carbon (mass = 12u):
Given mass / Molar mass = 62.4 / 12 = 5.2 moles
Moles of Hydrogen (mass = 1u):
Given mass / Molar mass = 4.19 / 1 = 4.2 moles (approx)
Moles of oxygen (mass = 16u):
Given mass / Molar mass = 33.2 / 16 = 2 moles
The ratio of moles of the respective elements in our compound:
(Moles of Carbon) : (Moles of Hydrogen) : (Moles of Oxygen)
5.2 : 4.2 : 2
Which can be written as:
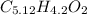
We are almost done but there is one problem, the formulas of compounds we see have whole numbers and not decimals
So, we will multiply all 3 of the numbers by the smallest number possible to get whole number ratios
Since there are 2 numbers we need to take care of and both of them have 2 in the decimal place,
we can multiply by 5 to get whole number ratios
So we get:
26 : 21 : 10
Therefore the molecular formula of the compound: