Answer:
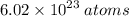
Step-by-step explanation:
since every compound is equivalent to one mole of particles. This number is represented by avagadros constant which represents the atomic proportion from molar to physical mass on a human scale. This equates to the amount of particles per mole of substance(amount of substance).
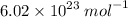
this can be denoted in symbol form by
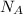
avagadros constant is defined as rational so the exact value is 6.02214076×10^23.
avagadros number : 1 mole