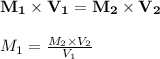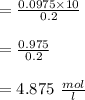Answer:
The answer is "
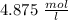 ".
".
Step-by-step explanation:
The molar absorptivity value is= 12.3
path length of cell = 1
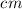
Absorbance = 1.2
Using the beer's lambert law:
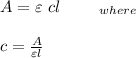
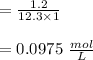
Claculating the concentration of the solution after dilution:
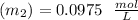
find out the dilution value before concentration :
The volume taken by the dilution:
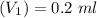
The final volume after dilution:
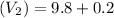
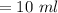
Formula: