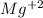Answer : The correct option is, (d)
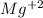
Explanation :
First we have to determine the number of electrons present in
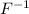 ion.
ion.
Atomic number of fluorine (F) = 9
As we know that,
Atomic number = Number of electrons (for neutral atom)
Number of electrons in
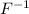 ion = 9 + 1 = 10
ion = 9 + 1 = 10
Now we have to determine the number of electrons present in given option ions.
For
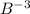 ion:
ion:
Atomic number of boron (B) = 5
Number of electrons in
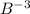 ion = 5 + 3 = 8
ion = 5 + 3 = 8
For
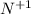 ion:
ion:
Atomic number of nitrogen (N) = 7
Number of electrons in
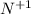 ion = 7 - 1 = 6
ion = 7 - 1 = 6
For
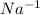 ion:
ion:
Atomic number of sodium (Na) = 11
Number of electrons in
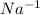 ion = 11 + 1 = 12
ion = 11 + 1 = 12
For
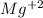 ion:
ion:
Atomic number of magnesium (Mg) = 12
Number of electrons in
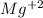 ion = 12 - 2 = 10
ion = 12 - 2 = 10
For
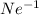 ion:
ion:
Atomic number of neon (Ne) = 10
Number of electrons in
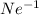 ion = 10 + 1 = 11
ion = 10 + 1 = 11
From this we conclude that magnesium ion,
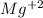 has the same number of electrons as a fluoride ion,
has the same number of electrons as a fluoride ion,
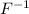 .
.
Hence, the correct option is, (d)