Answer:
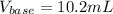
Step-by-step explanation:
Hello.
In this case, since the neutralization of the acid requires equal number of moles of both acid and base:

Whereas we can express it in terms of concentrations and volumes:
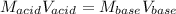
Thus, we can compute the volume of sodium hydroxide (base) as follows:
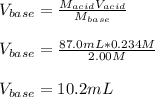
Best regards.