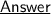
Let's get to the reaction first ~
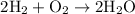
Here, we can infer that if we need 1 mole oxygen gas to react with 2 moles Hydrogen gas.
So, the number of Oxygen gas molecules we need to react should be half the number of Hydrogen gas molecules.
Number of Hydrogen gas molecules is :
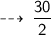
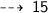
Number of Oxygen gas molecules needed is :
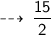
Number of Oxygen atoms needed :
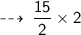
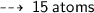
One atom of Oxygen weight 16 u, so 15 atoms weight ~
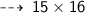
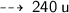
So, we need 240 u of Oxygen atoms to react completely with 30 u of Hydrogen atoms.