Answer:
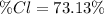
Step-by-step explanation:
Hello.
In this case, we can compute the percent composition of chlorine as shown below:
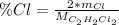
Whereas the atomic mass of chlorine is 35.45 g/mol and the molar mass of C₂H₂Cl₂ is 96.95 g/mol, thus, the percent composition turns out:
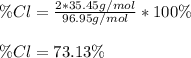
Best regards.