Answer:
110 grams
Step-by-step explanation:
The given chemical equation is
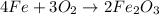
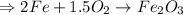
2 moles of Iron, Fe, react with 1.5 moles of oxygen,
 , to produce 1 mole of rust.
, to produce 1 mole of rust.
Or, 2x56=112 grams of Iron, Fe, reacts with 1.5x32=48 grams of oxygen,
 , to produce 112+48=160 grams of rust.
, to produce 112+48=160 grams of rust.
[As the mass of 1 mole of Fe is 56g and the mass of 1 mole of
 is 32g]
is 32g]
The given mass of Iron, Fe, is 100 g.
The given mass of the oxygen,
 is 33 g.
is 33 g.
Since 48 grams of oxygen required 112 grams of iron to react completely.
So, 1 gram of oxygen required 112/48 grams of iron to react completely.
So, 33 grams of oxygen required (112/48)x33=77 grams of iron to react completely.
Here, the availability of oxygen is less, so, the oxygen in the limiting agent.
So, the 33g of oxygen (reactant) will react with 77 g of iron (reactant) to produce 33+77=110 g of rust (product) as by the law of conservation of mass the mass of the product is the sum of masses of all the reactant. The remaining iron will remains be unreacted.