
As per the given equation, we can infer that :
For getting 2 moles mole of water, 1 mole of oxygen gas was required. so to get 4 moles of water we need :
We just have to multiply the whole reaction by 2 on both sides, in order to get 4 moles of H2O on product side.
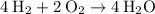
therefore, we need
