Answer:
The enthalpy change of the water during the process is 1.4 kJ/kg
Step-by-step explanation:
The enthalpy change of the water during the process can be determined from
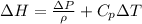
Where
 is the enthalpy change
is the enthalpy change
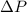 is the change in pressure
is the change in pressure
 is the density
is the density
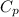 is the specific heat
is the specific heat
and
 is the temperature change
is the temperature change
From the question
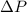 = 900 kPa - 100 k Pa
= 900 kPa - 100 k Pa
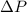 = 800 kPa
= 800 kPa
 = 1 kg/L = 1kg/dm³ = 1000 kg/m³
= 1 kg/L = 1kg/dm³ = 1000 kg/m³
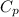 = 4.18 kJ/kg.°C
= 4.18 kJ/kg.°C
 = 0.15 °C
= 0.15 °C
∴
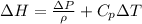
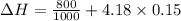
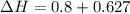
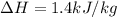
Hence, the enthalpy change of the water during the process is 1.4 kJ/kg