Answer: The mass is: "140 g " .
__________________________
Step-by-step explanation:
__________________________
Given: 6.0 g/ mL ; (the "density");
and the volume of the object: "24 mL" ;
We shall find the "mass" (measured in "g" ["grams"] :
__________________________
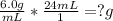 ;
;
__________________________
We can cancel out the "mL" units;
Since: "mL/mL" = 1 .
__________________________
and we have: " [6.0 * 24] g " ;
= 144 g ;
Now; since both given values in the problem have 2 (two) significant figures; and no "exact values" were given/used in this calculation;
we shall round our obtained calculation to: 2 (two) significant figures:
" 144 g" ; has 3 (three) significant figures ;
The first two significant figures are "14 ; and the following significant digit is "4" ; which is "less than 5" ; i.e, is either: "0, 1, 2, 3, or 4" ; so we round it down to "140" . {Note: If it were: 5, 6, 7, 8, or 9 ; then we would round the value up to "150".}.
__________________________
So: The mass is: "140 g " .
__________________________
Hope this helps! Wishing you the best!
__________________________