Answer:
The equilibrium constant of Fetal hemoglobin is 4 times larger than that of Adult hemoglobin
Step-by-step explanation:
From the question we are told that
The P50 value for Fetal hemoglobin is
![[P_(O_2)]_F = 19 torr](https://img.qammunity.org/2021/formulas/chemistry/high-school/nwcnkn579ewlbq7lpsf1s8rbpzkt0ngm01.png)
The P50 value for Adult hemoglobin is
![[P_(O_2)]_A = 26.8 torr](https://img.qammunity.org/2021/formulas/chemistry/high-school/taqp2b9k5f4ucc4jaig345ym2k3uic1uc2.png)
The chemical reaction for the binding process is
![4O_2_((g))+Hb_((aq))\to [Hb(O_2)_4_{{(aq)}}]](https://img.qammunity.org/2021/formulas/chemistry/high-school/23ud7hxh33e54o75qjwokbeysvycxz9a65.png)
Considering Fetal hemoglobin
Generally the equilibrium constant is mathematically represented as
![K_c_F = ([P_([Hb(O_2)_4)])/( [P_(O_2)]_F^4 * [P_(Hb)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/60bwmuse9jmyuof7k7dr3cwm4a9v1qjylz.png)
Here
![[P_([Hb(O_2)_4)] [\tex] and [P_(Hb)] will be 1 because both substances are aqueous</p><p>So</p><p> [tex]K_c_F = (1)/( 19^4 *1 )](https://img.qammunity.org/2021/formulas/chemistry/high-school/lz6k95zur62cc90yy7zxf8igd48ishhopg.png)
=>
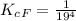
Considering Adult hemoglobin
Generally the equilibrium constant is mathematically represented as
![K_c_A = ([P_([Hb(O_2)_4)])/( [P_(O_2)]_A^4 * [P_(Hb)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/f2m94hcnmeoc0feymv4ywpglh35fm6ode4.png)
=>
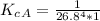
=>
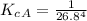
So the ratio of the equilibrium constant of Fetal hemoglobin to that of Adult hemoglobin is mathematically represented as
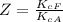
=>
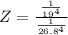
=>
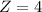
So the equilibrium constant of Fetal hemoglobin is 4 times larger than that of Adult hemoglobin