Answer:
1.50 × 10²⁴ atoms C
Step-by-step explanation:
Step 1: Define
Molar mass of C - 12.01 g/mol
Avagadro's Number: 6.02 × 10²³ atoms, molecules, formula units, etc.
Step 2: Use Dimensional Analysis
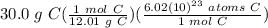 = 1.50375 × 10²⁴ atoms C
= 1.50375 × 10²⁴ atoms C
Step 3: Simplify
We are given 3 sig figs.
1.50375 × 10²⁴ atoms C ≈ 1.50 × 10²⁴ atoms C