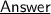
As per the given reaction, we can clearly observe that :
#1. How many Oxygen atoms are there in reactant and product side ?
- The number of Oxygen atoms is same as in reactant Side and product side, that is 6 atoms ~
#2. How does the reaction illustrate the law of conservation of mass ?
- According to law of conservation of mass, mass can neither be created nor be destroyed in a chemical reaction. so the mass of each element on reactant Side should be equal to that in the product side.
Now, let's check out if it's true for the given reaction ~
Mass on reactant side :
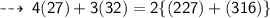
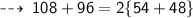
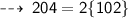
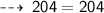
Hence, it's verified ~ that it follows law of conservation of mass ~