Answer:
(a)
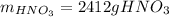
(b)
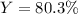
Step-by-step explanation:
Hello.
(a) In this case, by starting with 1216 grams of ammonia, we can firstly compute the yielded moles of NO in the first reaction considering the given yield as a fraction (0.962):
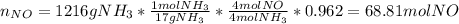
Next, in the second chemical reaction we compute the yielded moles of NO₂ with the 91.3-percent:
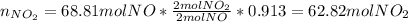
Finally, for the percent yield of the last chemical reaction and the molar mass of nitric acid (63 g/mol) we compute the yielded grams of nitric acid:
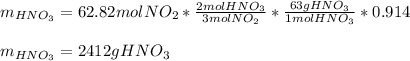
(b) In this case, we compute the moles of NO, NO₂ and the grams of nitric acid as well as the previous literal yet removing the percent yields since we are going to compute theoretical yields:
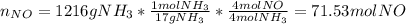
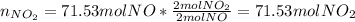
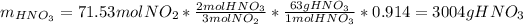
Thus, the overall percent yield is:
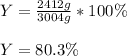
Best regards.