Answer:
The correct answer is - 75 grams.
Step-by-step explanation:
Half-life is the amount of time that is used by the given amount of substance or element to make it half of the initial amount of a particular radioactive substance.
Equation for alpha decay for radon
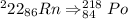
we need to calculate the left amount of radon after given time
using formula half life
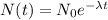
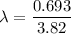
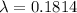
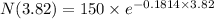
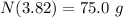
Thus, the left amount of radon is - 75 grams.