Answer:
volume = 1.04 dm^3
Step-by-step explanation:
Data:
volume = ?
Molarity, M = 0.25M
mass = 28.5g
Molar mass = 110.25g
Moles = ?
Solution:
First find number of moles,
moles = mass in gram / molar mass
= 28.5 / 110.25
= 0.258
≈ 0.26 moles
Now,
Molarity =
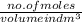
So,
Volume in dm^3 =
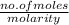
= 0.26 / 0.25
Volume = 1.04 dm^3