Answer:
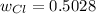
Step-by-step explanation:
Hello.
In this case, a partial chemical reaction can be written as:

Thus, the mass of chlorine in the initial seaborgium hexachloride (molar mass: 475.718 g/mol) is:
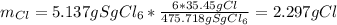
Which is also the total chlorine. Moreover, the chlorine from the HCl is:
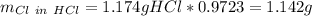
It means that the chlorine in the solid is:
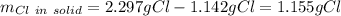
Therefore, the required fraction (w) is computed by dividing the mass of chlorine in the solid by the mass of chlorine in the initial seaborgium hexachloride as the only source of chlorine at the beginning:
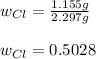
Best regards.