Answer:
We need to add 400 ml of pure water to obtain the solution at 55%
Explanation:
Concentration
The concentration of a solution measures the amount of solute that has been dissolved in a given amount of solution. The question states the alcohol is the solute and water is the solvent to produce the mix or solution.
The concentration can be calculated with the formula:
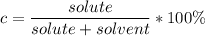
Originally, the mixture was 95% concentrated.
Substituting in the formula:
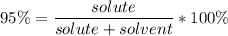
We know there were 550 ml of solution (solute+solvent), thus
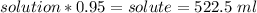
Now we know there were 522.5 ml of alcohol.
We now need to add x ml of water to have a 55% concentrated solution. Recall that water is solvent, the solute keeps its original value, thus:
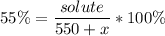
Operating:
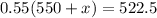
Dividing by 0.55
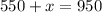
Thus
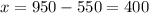
We need to add 400 ml of pure water to obtain the solution at 55%