Answer:
Step-by-step explanation:
Given that:
p = 6.17 × 10⁻³⁰ Cm
Electric field E = 94.7 × 10⁻⁶ N/C
Then:
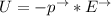
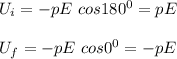
∴
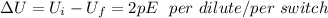
This implies that one switch of dipole produces 2pE energy to be deposited in the water.
mass of water = 13.1 g
The number of water molecule =
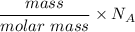
=
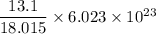
= 4.38 × 10²³ molecules of water.
∴ Number of dipoles = 4.38 × 10²³
The total number of switches in 2.39 sec = switch frequency × 2.73
Number of switches = 2.39 × 3.42 × 10⁸
Number of switches = 817380000
Number of switches = 8.174 × 10⁸
The total energy Q deposited = Energy deposited per dipole × Number of dipole × no of switches
Q = (2pE) × 4.38 × 10²³ × 8.174 × 10⁸
Q = (2 × 6.17 × 10⁻³⁰ × 94.7 × 10⁻⁶ × 4.38 × 10²³ × 8.174 × 10⁸ )
Q = 0.41838 J