Answer:
The mass flowrate of the cooling water needed to meet the regulation is 560 kg/s
Step-by-step explanation:
The given information are;
The mass flow rate of the water = 562.0 kg/s
The temperature of the exit water = 330.0 K
The allowable temperature of the of released water into nearby river = 310.00 K
The temperature of the cool water with which the temperature of the exit water is cooled = 290.00 K
Therefore, we have;
Heat gained by the mixture = The heat of the final mixture exited to the river
Q = m×c×ΔT
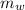 = 562.0 kg/s
= 562.0 kg/s
The mass of the cooling water = x
The final temperature = 310 K
4.2×562×(330 - 310) = x×4.2×(310 - 290)
47208 = 84×x
x = 47208/84 = 560 kg
Therefore, the mass flowrate of the cooling water needed to meet the regulation = 560 kg/s.