Final answer:
To calculate the number of carbon atoms in 11g of carbon dioxide, we determine the number of moles in 11g of CO2 and then multiply by Avogadro's number. This shows that 11g of CO2 contains 1.5055 ×
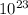 carbon atoms.
carbon atoms.
Step-by-step explanation:
The question asks us to calculate the number of carbon atoms (C atoms) in 11g of carbon dioxide (CO2). We'll use the molar mass of carbon and the molar mass of CO2 to find the answer.
The molar mass of CO2 is 44.009 g/mol, which includes 12.011g due to carbon. This means that the amount of carbon in 44.009 g of CO2 is just the molar mass of carbon, so 12.011 g of CO2 contains 1 mole of carbon or 6.022 ×
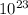 C atoms.
C atoms.
First, let's find out how many moles of CO2 we have in 11g:
Number of Moles = mass / molar mass = 11g / 44.009g/mol = 0.25 moles of CO2
Since we know that 1 mole of CO2 has 6.022 ×
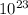 atoms of carbon, we can then calculate the number of C atoms in 0.25 moles:
atoms of carbon, we can then calculate the number of C atoms in 0.25 moles:
Number of C atoms = 0.25 moles × 6.022 ×
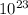 atoms/mol
atoms/mol
Therefore:
Number of C atoms = 1.5055 ×
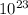 atoms
atoms