Answer:
2.46 × 10²⁵ atoms H
Step-by-step explanation:
Avagadro's Number: 6.022 × 10²³
Step 1: Find formulas
Hydrogen - H
Ammonium Sulfide - (NH₄)₂S
Step 2: Use Dimensional Analysis
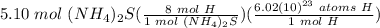 =
=
Step 3: Simplify
We have 3 sig figs.
2.45616 × 10²⁵ atoms H ≈ 2.46 × 10²⁵ atoms H