Answer:
11.23 atm
Step-by-step explanation:
Given
Mass = 13.7 g
Volume = 500mL = 0.5 L
Molar concentration =
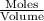
Moles =
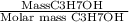 =
=
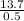 = 0.2279534 moles
= 0.2279534 moles
Molar concentration =
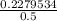 = 0.4559 M
= 0.4559 M
π = icRT
where
Osmotic pressure = π
Van't Hoff factor (i) = 1
Molar concentration of solute (c) = 0.4559 M
Ideal gas constant (R) = 0.0821 L.atm/K.mol
Kelvin Temperature (T) = 273 + 27 = 300 K
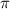 = 1 * 0.4559 * 0.0821 * 300
= 1 * 0.4559 * 0.0821 * 300
= 11.23 atm