Answer:
1. Given that the wavelength of the photon required to promote an electron in the hydrogen atom from the n = 1 to the n = 3 level is lt the wavelength of the photon required to promote an electron in the hydrogen atom from the n = 1 to the n = 2 level.
2. Given that the energy of a photon with a wavelength of 463 nm is gt the energy of a photon whose wavelength is 722 nm.
3. Therefore, in order to promote an electron to go to a higher energy level, light with a wavelength that is lt 400 nm is required
Step-by-step explanation:
The equation for electron transition in a hydrogen atom is given by the Rydberg equation as follows;
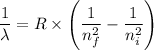
The energy required for electron transition is given by the formula;
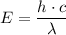
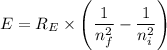
Where;
h = The Planck's constant
λ = The wavelength of light
c = The speed of light
n = Specific energy level
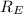 = -2.178 × 10⁻¹⁸ J
= -2.178 × 10⁻¹⁸ J
Therefore, the energy required to move an electron from one energy level to a higher energy level is inversely proportional to the wavelength of light, λ.
Increase in λ, results in lower energy available to transition to a higher energy level while a decrease in λ results in more energy available to transition to a higher energy level.