Answer:
E = 1.89 eV , E = 2.56 eV , E = 2.86 eV
Step-by-step explanation:
The emission of light in the visible range, explained by the Balmer series with expression
1 /λ=
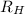 (1/2² - 1 / n²)
(1/2² - 1 / n²)
n = 3, 4, 5 ...
the constant R_{H} called Rydberg's constant and is equal to 1,097 10⁷ m⁻¹
These transitions are clearly explained by Bohr's atomic model, where the empirical series of Balmer and Rydberg are deduced from a theoretical model of the hydrogen atom in natural form.
Let's calculate the wavelengths for each transition
State
initial final λ (10⁻⁷ m)
3 2 6.5634
4 2 4.8617
5 2 4.3408
Let's calculate the energy of each of these wavelengths using Planck's equation
E = h f = h c /λ
λ = 6.5634 10⁻⁷ m
E = 6.63 10⁻³⁴ 3 10⁸ / 6.5634 10⁻⁷
E = 3.03 10⁻¹⁹ J
we reduce to eV
E = 3.03 10⁻¹⁹ J (1 eV / 1.6 10⁻¹⁹ J)
E = 1.89 eV
λ = 4.8617 10⁻⁷m
E = 6.63 10⁻³⁴ 3 10⁸ / 4.8617 10⁻⁷
E = 4.09 10⁻¹⁹ J
E = 2.56 eV
λ= 4.3408 10⁻⁷ m
E = 6.63 10⁻³⁴ 3 10⁸ / 4.3408 10⁻⁷
E = 4.582 10⁻¹⁹J
E = 2.86 eV