Answer:
Step-by-step explanation:
Given information:
A designed dialysis unit consists of a large no of small hollow fibers,
Considering the one fiber,
By using the volume of blood, we have :
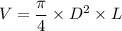
where:
L = 30cm = (30× 10) mm = 300 mm
Volume = 80 ml = 80 × 10³ mm
From the above equation, making D the subject, we have:

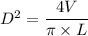
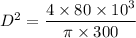
D² = 339.53
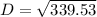
D = 18.43 mm
D = 1.843 cm
However, suppose we consider the laminar flow, the pressure loss in a single fiber can be determined as follows:
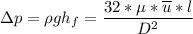
where;
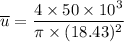
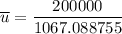
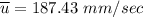
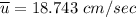
Recall:
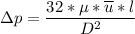
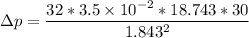
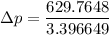
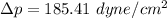
Finally, the number of needed to be used =
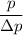
=
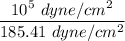
= 539.35