Here is the missing part of the question.
4. A distance runner is in a 4-hour race and sweats at a rate of 455 mL/hour. The sodium ion concentration in sweat is, on average, 920. mg/L. How many moles of sodium ions were lost during the race? Report your answer to three significant figures.
Answer:
Step-by-step explanation:
Given that:
the time for the runner = 4 hours
the rate of sweating = 455 mL/hour
Therefore, the total sweating rate = 455 mL/hour × 4 hours
the total sweating rate = 1820 mL
The
 conc. in sweat = 920 mg/L
conc. in sweat = 920 mg/L
Thus, the total number in grams of
 sweat lost =
sweat lost =
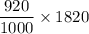
= 1674.4 mg
= 1.6744 grams
number of moles of
 sweat lost = mass of
sweat lost = mass of
 sweat lost/ molar mass of
sweat lost/ molar mass of

number of moles of
 sweat lost = 1.6744/ 23
sweat lost = 1.6744/ 23
number of moles of
 sweat lost = 0.0728 mole
sweat lost = 0.0728 mole
5. concentration of the runner = 142 mmol/L
the average volume of blood = 5.00 L
the number of moles sodium
 = concentration × volume
= concentration × volume
the number of moles sodium
 = 142 mmol/L × 5.000 L
= 142 mmol/L × 5.000 L
the number of moles sodium
 = 710 mmol
= 710 mmol
the number of moles sodium
 = 0.710 mol
= 0.710 mol
recall that; the number of moles of
 sweat lost = 0.0728 mol
sweat lost = 0.0728 mol
thus, the number of remaining
 = (0.71 - 0.0728) mol
= (0.71 - 0.0728) mol
the number of remaining
 = 0.6372 mol
= 0.6372 mol
The runner ending
 concentration = 0.6372 mol/ 5.00 L
concentration = 0.6372 mol/ 5.00 L
The runner ending
 concentration = 127440 mmol/L
concentration = 127440 mmol/L