Answer:
The sign of H is positive, and the products have more potential energy than the reactants.
Step-by-step explanation:
The options you were given are the following:
- The sign of H is positive, and the products have less potential energy than the reactants.
- The sign of H is positive, and the products have more potential energy than the reactants.
- The sign of H is negative, and the products have less potential energy than the reactants.
- The sign of H is negative, and the products have more potential energy than the reactants.
An endothermic reaction is a chemical reaction that absorbs heat from the surroundings. The absorbed energy provides the activation energy for the reaction to occur.
The sign of H refers to the change in enthalpy. Enthalpy is the thermodynamic property of a system. It is the sum of the internal energy added to the product of the pressure and volume of the system:
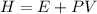
If the pressure and volume are constant, the change in enthalpy equals the transferred heat (q). If q is positive, we have an endothermic reaction. The change in enthalpy (the sign of H) in that case always positive, as the change in internal energy is positive (heat is absorbed).