Answer:
0.9375 grams
Step-by-step explanation:
As we know half life is the time period in which quantity of a substance reduces to half of its original value.
The formula to calculate amount of substance left after n half life is
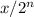
where x is the amount of original substance
n is the no of half life.
_________________________________________
Given
No. of half lives = 4
amount of original substance = 15 gram
thus,
n = 4
x = 15 grams
amount of radioisotope left after 4 half life =
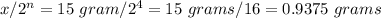
Thus, only 0.9375 grams of radioisotope are left after 4 half lives.