Answer:
8,95
Step-by-step explanation:
Density is equal to mass/volume
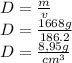
approximately
Curiosity:
Did you knew that is because of his low density that ships can fluctuate?
Despite their big mass, their volume are far greatter, so the Buoyancy Force acts heavily on them.