Answer:
C. Object B, by 1 gram per cubic centimeter.
Explanation:
Density =
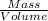
Volume x Density =
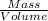 x Volume (cross both of volumes out)
x Volume (cross both of volumes out)
Volume x Density = Mass
So, what you would do is divide the Mass and Volume, and the density would be the outcome.
Since we see it has a mass of 12g and a volume of
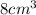
We would then do
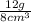 , this gives us 1.5, and
, this gives us 1.5, and
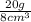 gives us 2.5, so that would give us the answer C.
gives us 2.5, so that would give us the answer C.