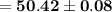Answer:
The answer is "
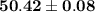 ".
".
Step-by-step explanation:
Overall delivered volume
![= [(49.06 \pm 0.05) + (1.77 \pm 0.05)]\ mL](https://img.qammunity.org/2021/formulas/chemistry/college/8rq1qtspha0jtgbac8v2r57kgn8p6pt69h.png)
Its blank solution without any of the required analysis
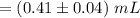
Compute the volume of the endpoint as follows:
Formula:
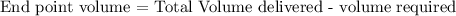
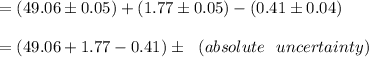
therefore,
absolute uncertainty
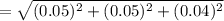

The Endpoint volume
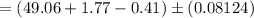
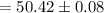
Therefore, the volume of the endpoint adjusted for the blank is: