Answer: m = 1710.35 pounds per hour
Step-by-step explanation: Since the step involves a gaseous state, it can be used the ideal gas formula, given by:
PV=nRT
For this question, R will be 8.31451 m³Pa/K.mol, which means the variables need a change of unit:
T = 330°C + 273 = 603K
P = 2.5atm*101325 = 253312.5Pa
V = 92.1ft³*0.0283 = 2.60643m³
Calculating mols:
PV=nRT
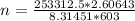
n = 131.7 mols
The reactant (propylene) spends 1.80 seconds in the tubes and 1 hour is 3600 seconds, so:
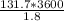 = 263377.5 mol/h
= 263377.5 mol/h
In the mixture, the proportion of propylene is 0.07, then:
263377.5*0.07 = 18436.4 mol of propylene in the mixture
Mol is related to mass and molar mass by the following formula:
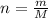
m = nM
Molar Mass of propylene is 42.08g/mol:
m = 18436.4*42.08 = 775803.712g
Changing into pounds:
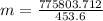
m = 1710.35lbs
It must be added 1710.35lbs per hour in the mixture.