Answer:
The energy is 22.592 kJ.
Step-by-step explanation:
Given that,
Mass of
 = 108.1 g
= 108.1 g
Molar mass = 18.01
Initial temperature = 65°C
Final temperature = 115°C
We need to calculate the energy
Using formula of energy
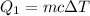
Put the value into the formula
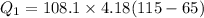
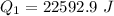
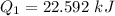
Hence, The energy is 22.592 kJ.