Answer:
0.179 mol H₂O
Step-by-step explanation:
STP - 1 atm, 0°C
Assuming we're in STP:
Step 1: Find conversions
1 mol = 22.4 L at STP
Step 2: Use Dimensional Analysis
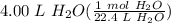 = 0.178571 mol H₂O
= 0.178571 mol H₂O
Step 3: Simplify
We have 3 sig figs.
0.178571 mol H₂O ≈ 0.179 mol H₂O