Answer:
The notation of configuration of Co is
![[Ar] 3d^7 4s^2](https://img.qammunity.org/2021/formulas/chemistry/college/hegddsqwt7g8ah15wtk4nogw4xer8m9ucu.png)
(A) is correct option.
Step-by-step explanation:
Given that,
The following notations is the correct noble gas configuration for Co.
(A). [Ar] 3d⁷ 4s²
(B). [Ar] 4s² 4p⁶ 3d
(C). [Kr] 4s² 4p⁶ 4d
(D). [Co] 3d⁷ 4s²
We know that,
Cobalt :
Co is the symbol of cobalt which is a chemical element.
Atomic number of Co is 27.
We need to find the notation of the configuration of noble gas Co
Using configuration
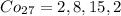
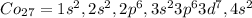
So, we can write as the notation of noble gas configuration
![Co_(27)=[Ar] 3d^7 4s^2](https://img.qammunity.org/2021/formulas/chemistry/college/vbilrgf3tomrsci3ljhvnqu1plrdr358wa.png)
Hence, The notation of configuration of Co is
![[Ar] 3d^7 4s^2](https://img.qammunity.org/2021/formulas/chemistry/college/hegddsqwt7g8ah15wtk4nogw4xer8m9ucu.png)
(A) is correct option.