Answer:
The answer is
0.38
Step-by-step explanation:
HCL is a strong acid and therefore undergo complete dissociation
We have
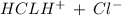
The concentration of Hydrogen ion is also 0.415 M
The pH of a solution can be found by using the formula
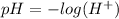
So we have
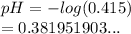
We have the final answer as
0.38
Hope this helps you