Answer:
The work transfer per unit mass is approximately 149.89 kJ
The heat transfer for an adiabatic process = 0
Step-by-step explanation:
The given information are;
P₁ = 1 atm
T₁ = 70°F = 294.2611 F
P₂ = 5 atm
γ = 1.5
Therefore, we have for adiabatic system under compression
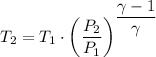
Therefore, we have;
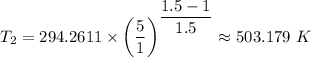
The p·dV work is given as follows;
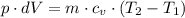
Therefore, we have;
Taking air as a diatomic gas, we have;
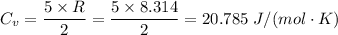
The molar mass of air = 28.97 g/mol
Therefore, we have
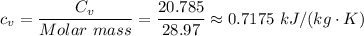
The work done per unit mass of gas is therefore;
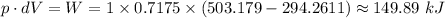
The work transfer per unit mass ≈ 149.89 kJ
The heat transfer for an adiabatic process = 0.