Answer:
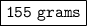
Step-by-step explanation:
The formula for density is:
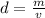
Rearrange the formula for mass, m. Multiply both sides by v.
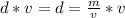
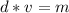
Mass is found by multiplying the mass by the volume.
The density of the bromine is 3.10 grams per cubic centimeter. The volume is 50 milliliters. Since a milliliter is equal to a cubic centimeter, the volume is also 50 cubic centimeters.
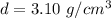
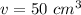
Substitute the values into the formula.
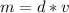
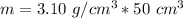
Multiply. Note the centimeters cubed will cancel out.
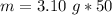
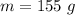
The mass of the bromine is 155 grams.