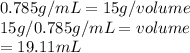Answer: The temperature is there to confuse you on purpose. Density changes with temp, but in these type of problems, they mean nothing
Step-by-step explanation:
The formula for density is mass per unit of volume.
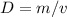
We dont have to do any conversions here so it boils down to division and multiplication.
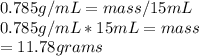
Now for the second part: