Answer:
2.1 × 10²⁴ atoms Pb
Step-by-step explanation:
Avogadro's number: 6.022 × 10²³
Step 1: Find conversions
1 mol Pb = 6.022 × 10²³ atoms Pb
Step 2: Use Dimensional Analysis
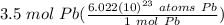 = 2.107 × 10²⁴ atoms Pb
= 2.107 × 10²⁴ atoms Pb
Step 3: Simplify
We have 2 sig figs.
2.107 × 10²⁴ atoms Pb ≈ 2.1 × 10²⁴ atoms Pb