Answer:
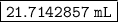
Step-by-step explanation:
The density formula is:
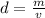
Rearrange this formula for v, volume. Multiply by v, then divide by d.
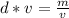
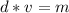
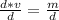
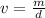
Volume can be found by dividing the mass by the density. The mass of the aluminum is 45.6 grams and the density is 2.1 grams per milliliter.
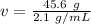
Divide. Note the grams, or g, will cancel out.
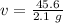
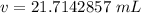
The volume of the aluminum is 21.7142857 milliliters.