Answer:
Option (b) "174 nm"
Step-by-step explanation:
Energy of emitted photon is 7.13 eV.
We need to find the wavelength of the photon.
1 eV = 1.6 × 10⁻¹⁹ J
Energy of a photon is given by :
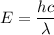
Where,
 is wavelength of the photon
is wavelength of the photon
Putting all the values,
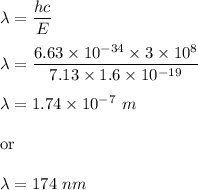
So, the wavelength of the photon is 174 nm.