Answer:
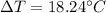
Step-by-step explanation:
Hello,
In this case, since the equation for computing the heat in terms of mass, specific heat and temperature change is:
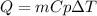
For the given heat and mass, and considering the specific heat of iron to be 0.444 kJ/(kg°C), the resulting temperature change is:
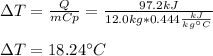
Best regards.