Answer:
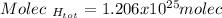
Step-by-step explanation:
Hello.
In this case, taking into account that HCl has one molecule of hydrogen per mole of compound which weights 36.45 g/mol, we compute the number of molecules of hydrogen in hydrochloric acid by considering the given mass and the Avogadro's number:
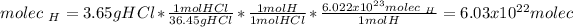
Now, from the 180 g of water, we see two hydrogen molecules per molecule of water, thus, by also using the Avogadro's number we compute the molecules of hydrogen in water:
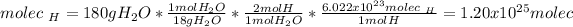
Thus, the total number of molecules turns out:
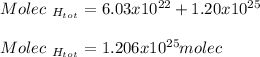
Regards.